skip to main |
skip to sidebar
Mitochondrion
This educational blog contains all the informations about Cell Mitochondrions.
Mitochondria is the semi-autonomous Cell Organelle.
Mitochondrion
Mitochondrial Science:
I Introduction

Mitochondria
Mitochondria, minute sausage-shaped structures found in the hyaloplasm (clear cytoplasm) of the cell, are responsible for energy production. Mitochondria contain enzymes that help to convert food material into adenosine triphosphate (ATP), which can be used directly by the cell as an energy source. Mitochondria tend to be concentrated near cellular structures that require large inputs of energy, such as the flagellum, which is responsible for movement in vertebrate sperm cells and single-celled plants and animals.
Mitochondria, generally physically independent substructures, one of several types of organelle (cell organ), found in almost all eukaryotic cells where they perform important functions, especially respiration and provision of energy in a form that cells can use.
The name mitochondria comes from two Greek words, “mito” meaning “filaments” and “chondros” meaning “grains”, which were used in the 19th century to describe the appearance of parts of cells under the light microscope. The number of mitochondria per cell may depend on the cell type. It was thought for a long time that a cell contained many (even thousands or more) mitochondria, typically sausage-shaped and of a similar size to bacteria, which were located independently of other cell components. Currently it is thought that, at least in some cells, the mitochondria comprise a largely connected and continuous single network which can sometimes interact with a second type of organelle, the endoplasmic reticulum. The extent to which there are many independent mitochondria in any given cell type, as accepted for many years and suggested by looking with a technique called electron microscopy at sections of cells, is currently being evaluated.
II Formation Of The Electron Donors NADH And FADH2
Through a series of metabolic reactions carried out in the matrix, the mitochondrion converts products of the cell's initial metabolism of fats, amino acids, and sugars into the compound acetyl coenzyme A. The acetate portion of this compound is then oxidized in a chain reaction called the tricarboxylic acid cycle. At the end of this cycle the carbon atoms yield carbon dioxide and the hydrogen atoms are transferred to the cell's most important hydrogen acceptors, the coenzymes nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD), yielding NADH and FADH 2. It is the subsequent oxidation of these hydrogen acceptors that leads eventually to the production of ATP.
NADH and FADH2 are compounds of high electron-donating capacity. Were they to transfer their electrons directly to oxygen, the resulting combustion would release a lethal burst of heat energy. Instead, the energy is released in a series of electron donor-acceptor reactions carried out, within the cristae of the mitochondrion, by a number of proteins and coenzymes that make up the electron-transport, or respiratory, chain.
III Mitochondrial DNA
Mitochondria contain their own DNA (deoxyribonucleic acid; see Nucleic Acids) (often called mtDNA). This mtDNA is much smaller than, and completely separate from, the nuclear DNA. mtDNA tends to be larger the more primitive the eukaryote; it provides information for a small number of mitochondrial proteins and nucleic acids. mtDNA is inherited maternally (see Heredity), hence analysis of mtDNA allows evolutionary relationships to be deduced (see Evolution). Use of this approach has suggested (controversially) that humans may have evolved 100,000 years ago from a region in Africa; it also establishes that Neanderthal man was not a precursor of today's Homo sapiens but belonged to a now extinct evolutionary line that had branched earlier.
IV Mitochondrial Origin
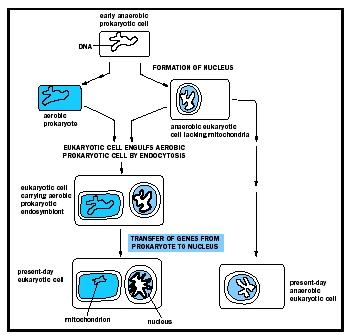
The presence of a small amount of mtDNA is one reason for supposing that mitochondria arose endosymbiotically (symbiotically—that is, in a mutually beneficial relationship—inside another organism) as a result of invasion of a primitive bacterium into a proto-eukaryotic cell; the mtDNA would be a relic of the original bacterial DNA. Lynn Margulis first postulated this serial endosymbiotic theory in 1970, and refined it in 1981. Other reasons for adopting this view include molecular analysis of mitochondrial nucleic acids and proteins; these often have resemblance to bacterial counterparts. It is postulated that mtDNA has shrunk with increasing complexity of cell type as information coded for by the DNA has been transferred to the nucleus, hence the larger mtDNA in more primitive eukaryotes. Although the protozoon Reclinomonas americana (a relatively primitive eukaryotic cell) does not have an unusually large mtDNA for a primitive eukaryote, recent investigations have shown that it codes for more proteins, all with close bacterial counterparts, than any other characterized mtDNA; this finding gives further impetus to the endosymbiotic theory. The exact basis for the reduction of the size of mtDNA as the complexity of the eukaryotic cell type increases, for example, from a fungus to a mammal, is not clear.
Mitochondria must divide in order to match cell division (see Meiosis; Mitosis). How exactly the right number (which would be two if there is only one mitochondrion per cell—see above) of mitochondria is achieved at the point of cell division is not known, but there is evidence that division is related to the process whereby bacterial cells divide. Again, this would be consistent with the endosymbiotic hypothesis of the origin of mitochondria.
V The Electron-Transport Chain
The proteins of this chain are embedded in the cristae membrane, actually traversing the lipid bilayer and protruding from the inner and outer surfaces. The coenzymes are dissolved in the lipid and diffuse through the membrane or across its surface. The proteins are arranged in three large complexes, each composed of a number of polypeptide chains. Each complex is, to continue the hydraulic analogy, a lock in the waterfall of the electron flow and the site at which energy from the overall redox reaction is tapped. The first complex, NADH dehydrogenase, accepts a pair of electrons from the primary electron donor NADH and is reduced in the process. It in turn donates these electrons to the coenzyme ubiquinone, a lipid-soluble molecule composed of a substituted benzene ring attached to a hydrocarbon tail. Ubiquinone, diffusing through the lipid of the cristae membrane, reaches the second large complex of the electron-transport chain, the b-c2 complex, which accepts the electrons, oxidizing ubiquinone and being itself reduced. (This complex can also accept electrons from the second primary electron donor, FADH2, a molecule below NADH in the electron-donating scale.) The b-c2 complex transfers the pair of electrons to cytochrome c, a small protein situated on the outer surface of the cristae membrane. From cytochrome c, electrons pass (four at a time) to the third large complex, cytochrome oxidase, which, in the final step of the chain, transfers the four electrons to two oxygen atoms and two protons, generating two water molecules.
This transfer of electrons, from member to member of the electron-transport chain, provides energy for the synthesis of ATP through an indirect route. At the beginning of the electron-transport chain, NADH and FADH2 split hydrogen atoms into protons and electrons, transferring the electrons to the next protein complex and releasing the protons into the mitochondrial matrix. When each protein complex in turn transfers the electrons down the chain, it uses the energy released in this process to pump protons across the inner membrane into the intermembrane space. (For the dynamics of this pumping action, see above The plasma membrane: Transport across the membrane.) This transport of positively charged protons into the intermembrane space, opposite the negatively charged electrons in the matrix, creates an electrical potential that tends to draw the protons back across the membrane. A high concentration of protons outside the membrane also creates the conditions for their diffusion back into the matrix. However, as is explained above, the inner membrane is extremely impermeable to protons. In order for the protons to flow back down the electrochemical gradient, they must traverse the membrane through transport molecules similar to the protein complexes of the electron-transfer chain. These molecules are the so-called F1F0ATPase, a complex protein that, transporting protons back into the matrix, uses the energy released to synthesize ATP. The protons then join the electrons and oxygen atoms to form water. (For further discussion of ATP production, see above The nature and function of cells: The cell as a collection of self-replicating catalysts: Coupled chemical reactions.)
This complex chain of events, the basis of the cell's ability to derive ATP from metabolic oxidation, was conceived in its entirety by the British biochemist Peter Mitchell in 1961. The years following the announcement of his chemiosmotic theory saw its ample substantiation and revealed its profound implications for cell biology.
VI Structure And Function
Structure of a Mitochondrion
This interactivity provides detailed information on the structure of a mitochondrion.
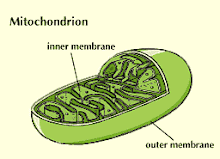
Mitochondria have two membranes, inner and outer. The latter serves largely as an outer boundary and is not the locus of major mitochondrial functions, whereas the inner membrane contains many proteins with important functions, including those that allow molecules to enter into, or exit from, the mitochondrial matrix.
The internal aqueous phase of mitochondria is known as the matrix. Apart from the mtDNA, many proteins are located in the matrix. Prominent is the set of catalysts (enzymes) for a series of reactions known as the Krebs' cycle or tricarboxylic acid cycle (see also Aerobic Respiration). This cycle receives a chemical unit known as the acetyl group that is derived from the breakdown of sugars (see Sugar Metabolism), fats, and proteins by cells. Precursors of the acetyl group are transported from the cell cytoplasm into the matrix via some of the proteins in the inner membrane. The reactions of the Krebs' cycle abstract electrons from the acetyl group and transfer them to two kinds of molecule that can subsequently pass them on to the electron transport chain that is described below. The two carbon atoms are converted into carbon dioxide, which is usually released from the cell.
VII The Chemiosmotic Theory
The chemiosmotic theory has four postulates:
(1) The inner mitochondrial membrane is impermeable to protons, hydroxide ions, and other cations and anions. This postulate was validated when it was shown that substances allowing protons to flow readily across mitochondrial membranes uncouple oxidative electron transport from ATP production.
(2) Transfer of electrons down the electron-transport chain brings about pumping of protons across the inner membrane, from matrix to intermembrane space. This was demonstrated in the laboratory by a reconstitution of components of the electron-transport chain in artificial membrane vesicles. The stimulation of electron transport caused a measurable buildup of protons within the vesicle.
(3) The flow of protons down a built-up electrochemical gradient occurs through a proton-dependent ATPase, so that ATP is synthesized from ADP and Pi whenever protons move through the enzyme. This hypothesis was confirmed by the discovery of what came to be known as the F1F0ATPase. Shaped like a knob attached to the membrane by a narrow stalk, F1F0ATPase covers the inner surface of the cristae. Its stalk (the F0 portion) penetrates the lipid bilayer of the inner membrane and is capable of catalyzing the transport of protons. The knob (the F1 portion) is capable of synthesizing as well as splitting, or hydrolyzing, ATP. F1F0ATPase is therefore reversible, either using the energy of proton diffusion to combine ADP and Pi or using the energy of ATP hydrolysis to pump protons out of the matrix.
(4) The inner membrane of the mitochondrion possesses a complement of proteins that bring about the transport of essential metabolites. Numerous carrier systems have been demonstrated to transport into the mitochondrion the products of metabolism that are transformed into substrates for the electron-transport chain. Best known is the ATP–ADP exchange carrier of the inner membrane. Neither ATP nor ADP, being large, charged molecules, can cross the membrane unaided, but ADP must enter and ATP must leave the mitochondrial matrix in order for ATP synthesis to continue. A single protein conducts the counter-transport of ATP against ADP, the energy released by the flow of ATP down its concentration gradient being coupled to the pumping of ADP up its gradient and into the mitochondrion.
VIII Function:
The mitochondrial matrix also contains enzymes for catalysing other important cell reactions. For example, the mitochondrion is the location for synthesis of the pigment haem found, for instance, in the oxygen-carrying protein haemoglobin, while the mitochondria of liver cells in terrestrial animals catalyse the synthesis of urea, a molecule whose excretion from the body removes unwanted nitrogen. This example illustrates that whereas mitochondria from different cell types have many features in common, they also have different capabilities depending upon the cell type in which they reside. Thus plant mitochondria are involved in reactions not found in mammalian cells.

Oxidative Phosphorylation
On the inner membrane of the mitochondria particular molecules (cytochromes, flavins, and coenzyme Q) accept and donate electrons; these electron carriers therefore form an electron transport chain. When the electrons from the glycolysis products NADH and FADH2 are transported through this chain to be donated to oxygen—which reduces the oxygen (O2) to water (H2O)—a large amount of free energy in the form of ATP is produced.
The protein machinery that couples respiration, the uptake of oxygen, to energy provision is located in the inner membrane. The process is known as oxidative phosphorylation. Complex proteins, collectively known as the electron transfer (or transport) chain, in the inner membrane transfer to oxygen the electrons released from the acetyl group by the reactions of the Krebs' cycle. The oxygen is converted to water, which is thus, along with carbon dioxide, a product of respiration. The electron transfer process is energetically “downhill”, that is, overall it releases energy. In the mitochondria of some cells, for example, those of certain brown fat cells in mammals and rodents, this energy is given out as heat, but usually it is harnessed for the synthesis of adenosine triphosphate (ATP) from adenosine diphosphate (ADP) and phosphate. The ATP is synthesized on the matrix-facing side of the inner membrane and most of it is subsequently exported through the inner membrane to the cytoplasm where it is used in many cell processes, for example, driving contraction in muscle cells. The ADP that is thereby produced is imported back into mitochondria in exchange for the exported ATP.
The coupling of the transfer of electrons to oxygen to the synthesis of ATP is known as the process of oxidative phosphorylation. A chemiosmotic mechanism—a theory first postulated by Peter Mitchell, who won the Nobel Prize for Chemistry in 1978—accounts for the coupling of the electron transport to the synthesis of ATP. This mechanism involves the active transport outward of protons across the inner membrane, driven by the electron transfer. This creates a proton electrochemical gradient, a term that expresses the charge and proton concentration difference across the membrane, which drives the subsequent return of the protons across the inner membrane via the ATP synthesizing enzyme, known as the ATP synthase. Exactly how this synthase functions is of great interest and Paul D. Boyer and John E. Walker shared the 1997 Nobel Prize for work on this subject. It is currently thought that proton movement induces rotational movements in the enzyme that induce parts of it to force the synthesis of ATP from ADP and phosphate.
Some of the protein units of the electron transport system have their genetic information carried by mtDNA. Mutations in mtDNA can result in defective electron transfer proteins and hence cause human diseases. There is also a view that mutations in mtDNA occur more frequently than in the nuclear DNA and are a major contributor to the ageing process.
Mitochondria are often called the powerhouses of the cell and as such can be thought of as keeping the cells alive. Recently, it has been surprisingly proposed that mitochondria play a central role in the process of programmed cell death, known as apoptosis. This means that under certain specialized conditions a process is triggered in cells that leads to regulated degradation of cell materials. The exact role(s) of mitochondria in this process are still under debate, but underscore the fact that although mitochondria are physically distinct entities in the eukaryotic cell, their essential functions are not independent, but rather are fully integrated into both the life and death of cells.